Our Research
Prostacare has undertaken two clinical studies to understand the safety and efficacy of water electrolysis for the treatment of BPH.
| Feasibility of Prostacare System in BPH Subjects | Safety and Efficacy Study | |
|---|---|---|
| Objectives |
|
|
| Subjects |
|
|
| Method |
|
|
| Findings |
|
|
Highlights of Prostacare's research include:
Excellent Tolerability without General Anaesthesia
The mean VAS score after placement of the device was 3.4. This was the most painful portion of the treatment for most subjects. At the beginning of the treatment, as measured 5 minutes into treatment, the mean VAS stabilized at 3.2, suggesting the electrolysis portion of the treatment did not increase subject discomfort. This level of pain is maintained throughout the treatment.
Overall, throughout the duration of procedure, the average pain level does not exceed a 5, which would be considered moderate pain; this suggests that this treatment is very well tolerated by the participants.
A Safe Alternative to Current Therapies
There were no reported cases of Device or procedure related Serious Adverse Events at 3 months.
That includes:
- 100% preservation of erections
- 100% preservation of continence
- 100% preservation of ejaculation

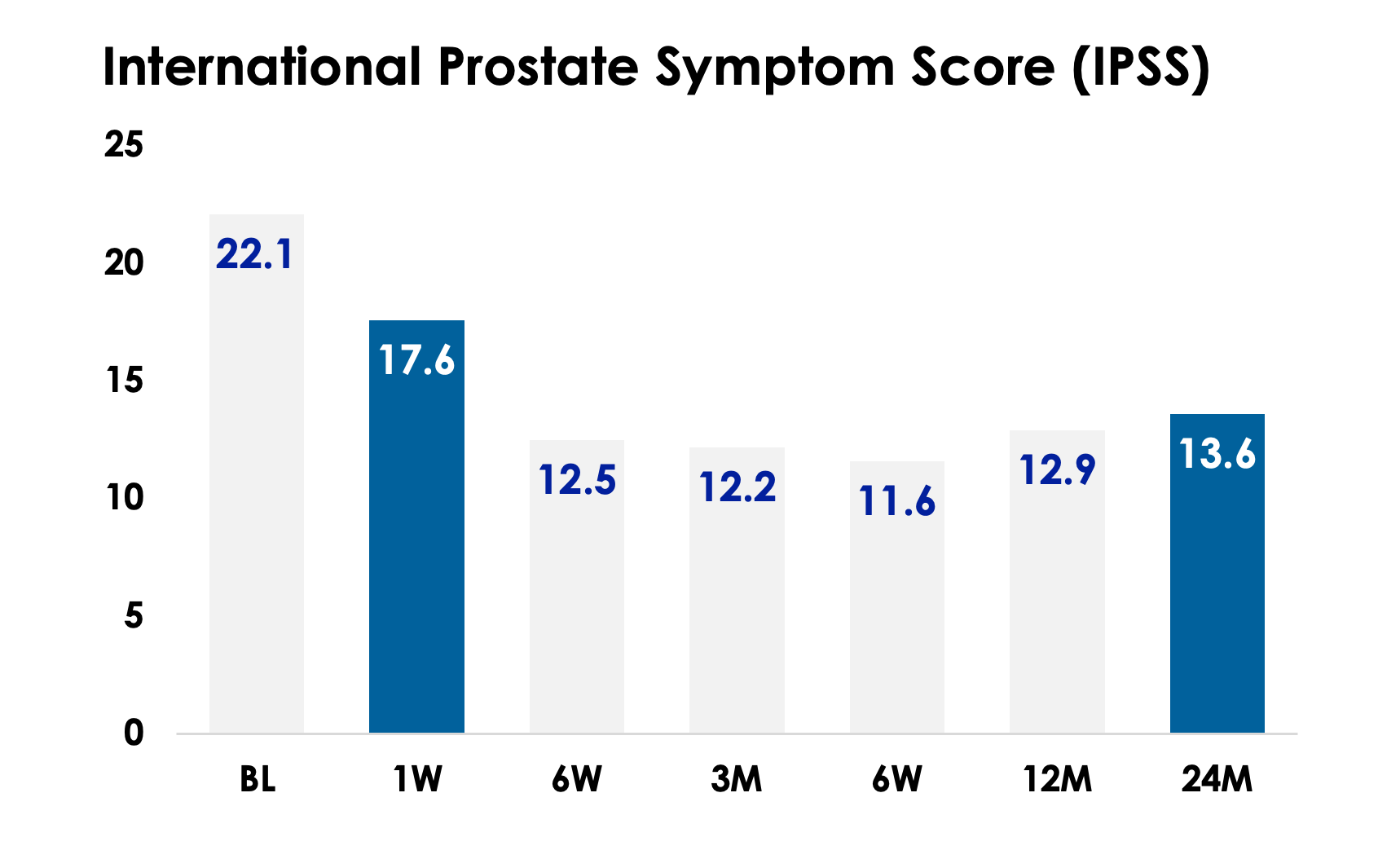
Lasting Relief in Days
The overall BPH symptom at baseline is “severe” with a mean IPSS of 21.7. At 1-week follow-up visit, there is a 4.9 (20.5%) IPSS score point decrease from baseline; this shows an overall BPH symptom change from “severe” to “moderate” within the first week of treatment. At 3-month follow-up visit subjects continued to experience improvement with a mean IPSS score change of 9.5 (43.9%) points compared to baseline.
Fast Treatment Time and Affordable Care
Treatment time is less than 30 minutes and patients can go home on the same day.
The treatment can be performed in the doctor's office, reducing costs to patients and practices.
Opportunities to Participate
You may be eligible to participate in one of Prostacare's upcoming BPH studies.
To learn more or to register your interest: Complete form

